It seems like I spend most of my life in a constant, never-ending battle against entropy, the tendency of all natural systems to progress toward a state of increasing disorder.
Yesterday, Shelly and I spent a good deal of time cleaning up the living room. I’ve moved a great deal of the stuff that used to be in my office into the apartment–eight years’ worth of business records, files, several computers (including a G4 Cube, arguably the most beautiful computer ever designed), and all the other stuff that accumulates when one spends time in one place for an extended period of time.
I hate entropy. The second law of thermodynamics says that energy will, whenever possible, seek to become disbursed. The net total sum of entropy in any closed system–such as, for example, the universe–always increases; stars burn out, iron rusts, people age and die.
And frankly, it all pisses me off.
It seems to me a damn poor way to run a universe–build into it an immutable natural law which guarantees, in the end, the heat death of that universe and everything in it.
Put most simply, entropy is energy, but it’s energy that can’t be used for anything. The total amount of entropy in a system is generally represented by the letter S; the relationship between entropy and energy is given by the equation
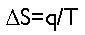
where delta-S is the change in entropy in the system, q is the amount of heat absorbed by the system, and T is the absolute temperature of the system at the time the heat was absorbed. As a system absorbs heat, the entropy of that system increases.
Certain processes in the universe are said to be “thermodynamically irreversible.” What that means, in its most basic form, is that certain changes in a system which result in increased entropy will not spontaneously reverse themselves. (It’s a bit more complex than that, of course; thermodynamically irreversible processes will reverse themselves, and that reversal itself is thermodynamically irreversible, if the environment changes in such a way that the reversal of the process increases entropy. But I digress.)
The notion of thermodynamic irreversibility is an important one. It means that, barring a change in the surrounding environment, certain processes once run will not revert back to their initial state. The universe tends toward a state of increasing entropy; a process that increases entropy isn’t going to go back on itself.
Consider a baseball lying on the ground. If you were to make a movie of how that baseball came to be lying on the ground–starting from a pitcher’s hand, flying toward a batter, striking the bat, zooming up into the air, falling back toward the ground–and then you were to play that movie backwards, you would not see anything that violates the laws of physics. The ball could start on the ground; then, all the air molecules around the ball could suddenly conspire to strike the ball in just exactly the right way to increase its kinetic energy, flinging it up from the ground and accelerating it toward the batter. The ball could strike the bat, where most of its energy would be absorbed by the bat and from there by the batter, the muscles in his arms taking that energy and dumping it into molecules of water and carbon dioxide, turning them into glucose molecules. Slowed and with its trajectory altered, the ball could go flying toward the pitcher, where his muscles absorbed the remaining kinetinc energy, turning it into chemical energy by combining water and carbon dioxide and storing the energy in the molecular bonds of the glucose his muscles created.
It could happen that way, but it doesn’t. This sequence of events would require a rather startling decrease in entropy–the random disorganized molecules of air suddenly conspiring together to move in just the right way to propel the ball, the ball-s trajectory taking it precisely back to the bat, the muscles in the player’s arms absorbing kinetic energy and storing it in glucose molecules…
It doesn’t happen that way because all these processes are thermodynamically irreversible. A ball flying through air will collide with the molecules in its way, which will absorb some of its energy and go rocketing off in random directions, generating heat and friction that slow the ball down; this increases entropy. The air molecules aren’t going to spontaneously move to a more highly ordered state, and push against the baseball in just the right way to speed it up; this process reduces entropy, and a reduction of entropy in one place can not happen unless it’s offset by a greater increase in entropy somewhere else.
My first exposure to entropy was in a college physics class, where we discussed the relationship between the ideal gas law, PV=nRT, and entropy. If you take a container of gas at high pressure, and you open the top of that container, the gas escapes until the pressure inside the container is equal to the pressure outside the container. If you open a container and leave it sitting in a room, the air in the room will not spontaneously start pouring into the bottle until the pressure inside the bottle is greater than the pressure outside the bottle. When the pressure inside and outside the bottle is equalized, the entropy in the system is at its maximum.
Each individual molecule of air is bouncing around totally at random, but the probability of all the molecules ending up in the bottle at the same time is very low indeed. As each molecule bounces around at random, the total net probability that any one molecule will be in any one place is pretty low, but the total net probability that the number of molecules in one place will be the same as the number of molecules in another is very high.
This is important, because there is a relationship between entropy and probability. In a gaseous system, if the system has two different states that it can be in, and the probability of the less probable state is given by P and the probability of the more probable state is given by P’, then the entropy in the system can be determined by
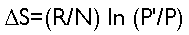
where R is the universal gas constant and N is the number of molecules of gas. More generally, in any kind of system, (R/N) will be replaced by some kind of constant, whose value depends on the particulars of the system.
That is, the entropy of a system increases when its state changes from a low probability condition to a high probability condition.
Now, let’s consider a universe without entropy. Entropy provides a mechanism by which certain changes are thermodynamically irreversible; the system favors one state over the other, and changes that increase entropy won’t spontaneously revert. But what happens without this mechanism?
All the structures that exist in the universe today are the result of the gradual accumulation of small changes over time. The initial state of the universe was uniform; the processes that caused non-uniformities to exist, that caused molecules of hydrogen to form, that caused those molecules to congregate and gravitate and form stars, that caused those stars to fuse heavier elements and burn out and explode, that caused the debris from these explosion to accumulate and form new stars…all these processes are thermodynamically irreversible. Some of these processes did result in spontaneous localized increases in order and decreases in entropy, to be sure; but the net sum total of entropy in the universe increased at every step. (Creationists always try to argue that thermodynamics means the spontaneous emergence of highly ordered living things, and the spontaneous increase in the complexity of those living things, is impossible; what the Creationists don’t get is that entropy always increases in a closed system, but this planet is not a closed system. Increase in order is permissible, if you add energy to the system; but adding energy to the system increases entropy somewhere else. The sun is an enormous maw of entropy; the decrease of entropy in living things here is more than offset by the increase in entropy there.)
So without entropy, there’s no mechanism for these small changes to accumulate. Without entropy, the changes that result inexorably in the formation of stars and planets and iPods and you and I aren’t irreversible; the system tends toward a steady state, with each change equally likely to be reversed.
So in other words, without entropy we wouldn’t be here. Without that ratchet that lets changes happen but then prevents them from un-happening, the universe doesn’t do anything interesting.
Which means I shouldn’t really resent entropy as much as I do. But dammit, it still seems like a poor way to run a universe to me. A universe that doesn’t run down and end in heat death, but doesn’t do anything interesting, or a universe that does marvelous and interesting things, then sputters out and dies…man, I want another choice!
*fans self* Has anyone mentioned how sexy it is when you talk like that?
*fans self* Has anyone mentioned how sexy it is when you talk like that?
We’re a young race. Don’t assume that just because we haven’t found a loophole yet doesn’t mean that none exist.
Sure, that doesn’t leave a whole lot of room for genuine hope, but it’s better than throwing one’s hands up and saying “screw it all!”. Besides, the time span between now and the heat death of the universe leaves room for an awful lot of intellectual pursuits, exploration, computer games, and fucking. 🙂
And, thankfully, entropy can be moved from place to place, which means that as long as we’re careful, we can keep finding places to dump entropy for a good long while. That ought to get us by for a long time, though in the end, I think we’re going to have to abandon this universe. Which presents a technical challenge, because it’s the only one we know about…
We’re a young race. Don’t assume that just because we haven’t found a loophole yet doesn’t mean that none exist.
Sure, that doesn’t leave a whole lot of room for genuine hope, but it’s better than throwing one’s hands up and saying “screw it all!”. Besides, the time span between now and the heat death of the universe leaves room for an awful lot of intellectual pursuits, exploration, computer games, and fucking. 🙂
Stop the world, I’m getting off!
But that’s the whole problem, isn’t it? Wherever you go, entropy follows you.
Stop the world, I’m getting off!
The current hope of escape is not to fix this one, but to find a way to transmit oneself through a wormhole into another universe that won’t burn out for another 10^65 years after the current one is done.
And yeah, not having entropy or not having it at the rate it operates here pretty much means a universe that’s just a soup of subatomic particles, or oooh…hey, maybe we can get as complex as helium!—but no further…but hey, it’s steady-state! That’s kind of what you were hoping for right?
re: transmit oneself through a wormhole into another universe
Or suppose that beings from another (dying) universe already discovered a means to travel through wormholes and there are some now occupying our own universe.
“The current hope of escape is not to fix this one, but to find a way to transmit oneself through a wormhole into another universe that won’t burn out for another 10^65 years after the current one is done.”
I have a feeling that transmitting one’s self through a wormhole means arriving at the destination in…err, disassociated condition.
The current hope of escape is not to fix this one, but to find a way to transmit oneself through a wormhole into another universe that won’t burn out for another 10^65 years after the current one is done.
And yeah, not having entropy or not having it at the rate it operates here pretty much means a universe that’s just a soup of subatomic particles, or oooh…hey, maybe we can get as complex as helium!—but no further…but hey, it’s steady-state! That’s kind of what you were hoping for right?
re: transmit oneself through a wormhole into another universe
Or suppose that beings from another (dying) universe already discovered a means to travel through wormholes and there are some now occupying our own universe.
“The current hope of escape is not to fix this one, but to find a way to transmit oneself through a wormhole into another universe that won’t burn out for another 10^65 years after the current one is done.”
I have a feeling that transmitting one’s self through a wormhole means arriving at the destination in…err, disassociated condition.
And, thankfully, entropy can be moved from place to place, which means that as long as we’re careful, we can keep finding places to dump entropy for a good long while. That ought to get us by for a long time, though in the end, I think we’re going to have to abandon this universe. Which presents a technical challenge, because it’s the only one we know about…
But that’s the whole problem, isn’t it? Wherever you go, entropy follows you.
You just rewrote The Last Question without the happy ending.
You know, the happy ending on that story always seemed a little implausible to me. It’s a deus ex machina in a very literal sense, and dammit, I just don’t buy it.
Most of what Asimov wrote seemed forced to me. Any decent league of Foundation Sociologists should have factored in a mutation like the Mule.
His plots were just too driven by his situations; but he did do it better than Arthur Can’t-Write-Believable-Characters Clarke.
You just rewrote The Last Question without the happy ending.
You know, the happy ending on that story always seemed a little implausible to me. It’s a deus ex machina in a very literal sense, and dammit, I just don’t buy it.
Most of what Asimov wrote seemed forced to me. Any decent league of Foundation Sociologists should have factored in a mutation like the Mule.
His plots were just too driven by his situations; but he did do it better than Arthur Can’t-Write-Believable-Characters Clarke.